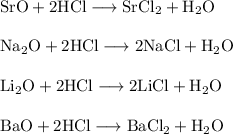Generally, metal oxides reacts with hydrochloric acid(HCl) to form the corresponding salts and water
SrO + 2HCl → SrCl2 + H2O
Na2O + 2HCl → 2NaCl + H2O
Li2O + 2HCl → 2LiCl + H2O
BaO + 2HCl → BaCl2 + H2O
Explanation:
Metal oxides are basic in nature .This means they have a high pH value ( >7 ). This is why if they react with acid the product becomes salt and water. Now let us write a balance equation between the following metal oxides and Hydrochloric acid(HCl) as stated in the questions.
SrO + 2HCl → SrCl2 + H2O
The above equation has the reactant at the left hand side and the products at the right hand side. Balancing the equation requires that the number of each element present on the reactant sides need to be equal on the product sides . Sr(strontium) has only one atom(SrO) and one atom of oxygen (SrO) on the reactant side. On the product side, Sr has one atom(SrCl) and one atom of oxygen(H2O). So Sr and O is balanced on both sides. The hydrogen on the product sides has 2 atom and the chlorine has 2 atom too . To balance the 2 atoms with that on the reactant side we add 2 in front of HCl to make hydrogen 2 atoms and chlorine 2 atoms.
Na2O + 2HCl → 2NaCl + H2O
Same method applied to balance the first equation is used here . Make sure every number of atom on the left side is equal to the right sides. The sodium is diatomic on the left and right sides. The oxygen has one atom on both sides .The hydrogen is diatomic on both sides and chlorine is diatomic on both sides. so the equation is balanced.
Li2O + 2HCl → 2LiCl + H2O
The equation is balance here as I added 2 in front of HCl to balance hydrogen and chlorine on both sides. And add 2 in front of LiCl to balance with the diatomic Chlorine and lithium on the reactant side.
BaO + 2HCl → BaCl2 + H2O
I only added 2 to the HCl acid to balance it with what we have on the right sides.