
Chemistry, 04.02.2020 09:49 greekfreekisdbz
The balanced equation for combustion in an acetylene torch is shown below:
2c2h2 + 5o2 → 4co2 + 2h2o
the acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many moles of co2 are produced when 35.0 mol c2h2 react completely?
mol co2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1


Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2
You know the right answer?
The balanced equation for combustion in an acetylene torch is shown below:
2c2h2 + 5o2 → 4co...
2c2h2 + 5o2 → 4co...
Questions


Mathematics, 23.04.2020 03:32


Mathematics, 23.04.2020 03:32



Mathematics, 23.04.2020 03:33

Physics, 23.04.2020 03:33

Chemistry, 23.04.2020 03:33


Mathematics, 23.04.2020 03:33



Mathematics, 23.04.2020 03:33

Mathematics, 23.04.2020 03:33

Business, 23.04.2020 03:33


Physics, 23.04.2020 03:33

Mathematics, 23.04.2020 03:33

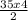 = 70mol
= 70mol

