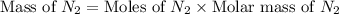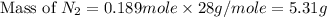What is the mass of 4.25 liters of n2 gas at stp?
3.91 grams 4.65 grams 4.96 grams 5.31 gram...

Chemistry, 27.09.2019 16:30 mateotrevino1
What is the mass of 4.25 liters of n2 gas at stp?
3.91 grams 4.65 grams 4.96 grams 5.31 grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Questions

Mathematics, 06.04.2022 14:00

Biology, 06.04.2022 14:00


Mathematics, 06.04.2022 14:00


Social Studies, 06.04.2022 14:00

Physics, 06.04.2022 14:00

SAT, 06.04.2022 14:00


Physics, 06.04.2022 14:00


Mathematics, 06.04.2022 14:00

Physics, 06.04.2022 14:00

SAT, 06.04.2022 14:00

Mathematics, 06.04.2022 14:00





Computers and Technology, 06.04.2022 14:00


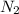 gas
gas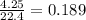 mole of
mole of