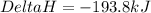Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
What is the heat of reaction when sulfur dioxide reacts with oxygen to form sulfur trioxide? 2so2(g...
Questions



Business, 02.12.2021 22:30



Chemistry, 02.12.2021 22:30



Computers and Technology, 02.12.2021 22:30

Law, 02.12.2021 22:30

Mathematics, 02.12.2021 22:30



English, 02.12.2021 22:30

Social Studies, 02.12.2021 22:30

History, 02.12.2021 22:30


Computers and Technology, 02.12.2021 22:30

Mathematics, 02.12.2021 22:30

Social Studies, 02.12.2021 22:30

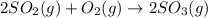
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0273/9627/76c37.png)
![\Delta H=[(n_{SO_3}\times \Delta H_{SO_3})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{SO_2}\times \Delta H_{SO_2})]](/tpl/images/0273/9627/23304.png)
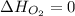 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(2\times -395.7)]-[(1\times 0)+(2\times -298.8)]](/tpl/images/0273/9627/57adc.png)