Which statement about the following reaction is correct?
cao (s) + h2o (l) > ca(oh...

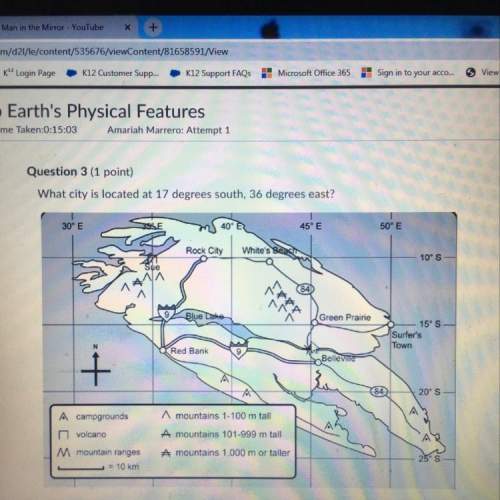
Chemistry, 15.11.2019 06:31 BlehBlehBlehBleh
Which statement about the following reaction is correct?
cao (s) + h2o (l) > ca(oh) 2 (s) deltah = -65.2 kj
65.2 kj of heat is absorbed for every one of cao that reacts.
130 kj of heat is released for every mole of h2o that reacts.
32.6 kj of heat is released for every 0.5 mole of cao that reacts.
16.3 kj of heat is absorbed for every 0.5 moles of h2o that reacts.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
Questions




Mathematics, 21.01.2021 09:40

Mathematics, 21.01.2021 09:40


English, 21.01.2021 09:40

Mathematics, 21.01.2021 09:40

Mathematics, 21.01.2021 09:40


English, 21.01.2021 09:40

Mathematics, 21.01.2021 09:40

English, 21.01.2021 09:40

History, 21.01.2021 09:40

Biology, 21.01.2021 09:40

Mathematics, 21.01.2021 09:40

Mathematics, 21.01.2021 09:40

English, 21.01.2021 09:40


Social Studies, 21.01.2021 09:40