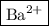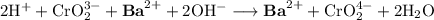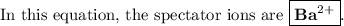2h+ + cro2 3- + ba2+ + 2oh- → ba2+ + cro2 4- + 2h2o

Chemistry, 10.01.2020 11:31 emmybear103002
Consider the total ionic equation below.
2h+ + cro2 3- + ba2+ + 2oh- → ba2+ + cro2 4- + 2h2o
what are the spectator ions in this equation?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Consider the total ionic equation below.
2h+ + cro2 3- + ba2+ + 2oh- → ba2+ + cro2 4- + 2h2o
2h+ + cro2 3- + ba2+ + 2oh- → ba2+ + cro2 4- + 2h2o
Questions


Mathematics, 24.03.2021 01:00

Biology, 24.03.2021 01:00

History, 24.03.2021 01:00


Spanish, 24.03.2021 01:00

History, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00

Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00

Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00

English, 24.03.2021 01:00

German, 24.03.2021 01:00

Mathematics, 24.03.2021 01:00