Chemistry, 04.01.2020 14:31 battlemarshmell
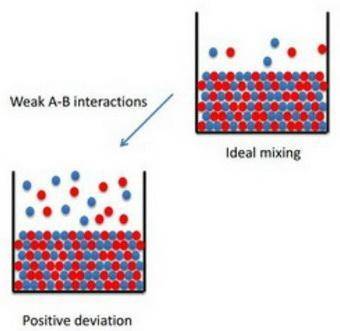
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. the resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. if, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. an actual vapor pressure greater than that predicted by raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by raoult's law is a negative deviation. part a imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. will this solution deviate positively from, deviate negatively from, or ideally follow raoult's law? '

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 to...
Questions

Chemistry, 27.04.2021 19:20


English, 27.04.2021 19:20

Geography, 27.04.2021 19:20


Mathematics, 27.04.2021 19:20


Mathematics, 27.04.2021 19:20


Chemistry, 27.04.2021 19:20

History, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Chemistry, 27.04.2021 19:20