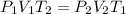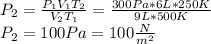Chemistry, 30.06.2019 07:20 redrhino27501
At a temperature of 500 kelvins, 6 liters of an ideal gas had a pressure of 300 newtons per square meter. if the temperature was reduced to 250 kelvins, and the volume raised to 9 liters, what was the resulting pressure ? (a) 100 newtons/m^2 (b) 150 newtons/m^2 (c) 450 newtons/m^2 (d) 900 newtons/m^2 (e) none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
At a temperature of 500 kelvins, 6 liters of an ideal gas had a pressure of 300 newtons per square m...
Questions

Mathematics, 15.11.2020 19:30


Chemistry, 15.11.2020 19:30




Mathematics, 15.11.2020 19:30

Mathematics, 15.11.2020 19:30

Mathematics, 15.11.2020 19:30

English, 15.11.2020 19:30

History, 15.11.2020 19:30

History, 15.11.2020 19:30

History, 15.11.2020 19:30

Mathematics, 15.11.2020 19:30

English, 15.11.2020 19:30