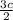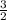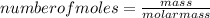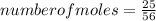Chemistry, 30.06.2019 06:40 saucydolphin20p6x92l
Consider the following reaction: iron (s) + chlorine (g) à iron (iii) chloride a. write the balanced chemical equation. b. 25.0 g of iron reacts with excess chlorine gas. a. calculate the moles of iron reactant. b. calculate the moles of iron (iii) chloride. c. calculate the mass of iron (iii) chloride.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
Consider the following reaction: iron (s) + chlorine (g) à iron (iii) chloride a. write the balance...
Questions

Social Studies, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01

Computers and Technology, 02.10.2020 23:01



Mathematics, 02.10.2020 23:01

Health, 02.10.2020 23:01

History, 02.10.2020 23:01


Mathematics, 02.10.2020 23:01


Geography, 02.10.2020 23:01


English, 02.10.2020 23:01



History, 02.10.2020 23:01


Chemistry, 02.10.2020 23:01