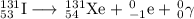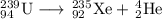Some radioactive nuclides have very short half-lives, for example, i-31 has a half-life of approximately 8 days. pu-234, by comparison has a half-life of 24,000 years. explain why both of these examples are dangerous, even though their half-lives are very different. be sure to describe the different major types of radiation, and their hazards. (radioactive decay and half-life)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Some radioactive nuclides have very short half-lives, for example, i-31 has a half-life of approxima...
Questions




Mathematics, 16.07.2019 18:50

Mathematics, 16.07.2019 18:50

Mathematics, 16.07.2019 18:50

Health, 16.07.2019 18:50


Arts, 16.07.2019 18:50

History, 16.07.2019 18:50


Mathematics, 16.07.2019 18:50


Chemistry, 16.07.2019 18:50




Mathematics, 16.07.2019 18:50


Biology, 16.07.2019 18:50