
Chemistry, 29.06.2019 20:40 bubba24640
Asample of cold water was mixed with a sample of hot water inside a calorimeter, and the final temperature of the cold water was 40 °c. which statement is true about the hot water in the calorimeter? it absorbed 40 joules. it absorbed 80 joules. its final temperature was 80 °c. its final temperature was 40 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
Asample of cold water was mixed with a sample of hot water inside a calorimeter, and the final tempe...
Questions



Mathematics, 13.05.2021 08:50


History, 13.05.2021 08:50


English, 13.05.2021 08:50

Arts, 13.05.2021 08:50



Mathematics, 13.05.2021 08:50

English, 13.05.2021 08:50


Mathematics, 13.05.2021 08:50

Mathematics, 13.05.2021 08:50


English, 13.05.2021 08:50

Mathematics, 13.05.2021 08:50

Biology, 13.05.2021 08:50

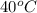 .
.


