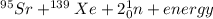Chemistry, 29.06.2019 14:10 phsycotic121
[oss.03]the models below represent nuclear reactions. the atoms on the left of the equal sign are present before the reaction and the atoms on the right of the equal sign are produced after the reaction. model 1: atom 1 + atom 2 = atom 3 + energymodel 2: atom 4 = atom 5 + atom 6 + energywhich of these statements is most likely correct about the two models? both models show reactions which use up energy in the sun. both models show reactions which produce energy in the sun. model 1 shows reactions in the sun and model 2 shows reactions in the nuclear power plants. model 1 shows reactions in the nuclear power plants and model 2 shows reactions in the sun.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
[oss.03]the models below represent nuclear reactions. the atoms on the left of the equal sign are pr...
Questions


English, 08.12.2021 23:50

Mathematics, 08.12.2021 23:50


History, 08.12.2021 23:50

History, 08.12.2021 23:50



English, 08.12.2021 23:50


Mathematics, 09.12.2021 01:00


Chemistry, 09.12.2021 01:00


Mathematics, 09.12.2021 01:00

Computers and Technology, 09.12.2021 01:00

Social Studies, 09.12.2021 01:00



Mathematics, 09.12.2021 01:00

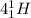 →
→ 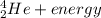 + some other particles
+ some other particles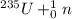 →
→