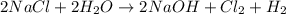Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
In the reaction 2nacl + 2h2o → 2naoh + c12 + h2, the reactants should: weigh more than the products...
Questions

Physics, 29.01.2020 10:55

English, 29.01.2020 10:55



History, 29.01.2020 10:55





Mathematics, 29.01.2020 10:55

Physics, 29.01.2020 10:55

Biology, 29.01.2020 10:55

Spanish, 29.01.2020 10:55

Social Studies, 29.01.2020 10:55

Biology, 29.01.2020 10:55

Mathematics, 29.01.2020 10:55


History, 29.01.2020 10:55


Chemistry, 29.01.2020 10:55