

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
Which of the following ions is in the lowest oxidation state? a. p in h2po−4 b. cr in cr2o2−7 c. fe...
Questions

Spanish, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

English, 19.09.2020 01:01


History, 19.09.2020 01:01

English, 19.09.2020 01:01

Business, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01



Mathematics, 19.09.2020 01:01

History, 19.09.2020 01:01





Mathematics, 19.09.2020 01:01

Chemistry, 19.09.2020 01:01


Biology, 19.09.2020 01:01

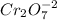
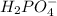 is +5
is +5![Cr_{2}O_{7} ]^{-2} \\](/tpl/images/0030/8970/f3e30.png) is
is 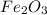 is +3
is +3 

