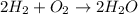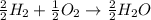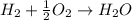Chemistry, 29.06.2019 11:20 dakotaadkins1818
Will mark ! read the chemical equation. 2h2 + o2 → 2h2o which of the following statements would be correct if one mole of hydrogen was used in this reaction? a. one mole of oxygen was used in this reaction. b. two moles of oxygen were used in this reaction. c. one mole of water was produced from this reaction. d. two moles of water were produced from this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Will mark ! read the chemical equation. 2h2 + o2 → 2h2o which of the following statements would be...
Questions


Mathematics, 03.02.2021 22:10


Mathematics, 03.02.2021 22:10


Mathematics, 03.02.2021 22:10



Mathematics, 03.02.2021 22:10

Mathematics, 03.02.2021 22:10

History, 03.02.2021 22:10

Mathematics, 03.02.2021 22:10


Mathematics, 03.02.2021 22:10


Chemistry, 03.02.2021 22:10


Mathematics, 03.02.2021 22:10

Mathematics, 03.02.2021 22:10