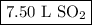Chemistry, 09.10.2019 10:50 dmead22284
When 7.50 l of sulfur trioxide are produced by the reaction of sulfur dioxide in an excess of oxygen at standard temperature and pressure, how many liters of sulfur dioxide were used? 2so2 (g) + o2 (g) yields 2so3 (g)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1


Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

You know the right answer?
When 7.50 l of sulfur trioxide are produced by the reaction of sulfur dioxide in an excess of oxygen...
Questions

Mathematics, 02.02.2020 17:02





Mathematics, 02.02.2020 17:02

Mathematics, 02.02.2020 17:02



Mathematics, 02.02.2020 17:02

Biology, 02.02.2020 17:02

Mathematics, 02.02.2020 17:02

History, 02.02.2020 17:02

History, 02.02.2020 17:02

Health, 02.02.2020 17:02

Mathematics, 02.02.2020 17:02

Social Studies, 02.02.2020 17:02

Mathematics, 02.02.2020 17:02

Computers and Technology, 02.02.2020 17:03