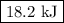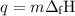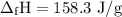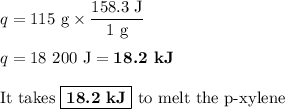Chemistry, 29.06.2019 05:00 lordcaos066
P-xylene, c8h10, has an enthalpy of fusion of 158.3 j g-1 and its melting point temperature is 13.2°c. how much heat is required to transform 115 g of solid p-xylene at 13.2°c into liquid p-xylene, also at 13.2°c?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
P-xylene, c8h10, has an enthalpy of fusion of 158.3 j g-1 and its melting point temperature is 13.2°...
Questions


Physics, 13.12.2021 21:20


Mathematics, 13.12.2021 21:20

Mathematics, 13.12.2021 21:20

Mathematics, 13.12.2021 21:20


Mathematics, 13.12.2021 21:20

Computers and Technology, 13.12.2021 21:20

Chemistry, 13.12.2021 21:20


Health, 13.12.2021 21:20



Biology, 13.12.2021 21:20

SAT, 13.12.2021 21:20

Biology, 13.12.2021 21:20

SAT, 13.12.2021 21:20

Physics, 13.12.2021 21:20