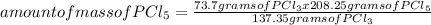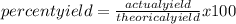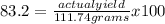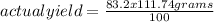Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
The percentage yield for the reaction pcl3 + cl2 → pcl5 is 83.2%. what mass of pcl5 is expected fro...
Questions



History, 17.12.2020 08:00

Health, 17.12.2020 08:00

Mathematics, 17.12.2020 08:00


Mathematics, 17.12.2020 08:00


Mathematics, 17.12.2020 08:00

Mathematics, 17.12.2020 08:00


Mathematics, 17.12.2020 08:00




English, 17.12.2020 08:00


Spanish, 17.12.2020 08:00

Mathematics, 17.12.2020 08:00

Chemistry, 17.12.2020 08:00