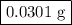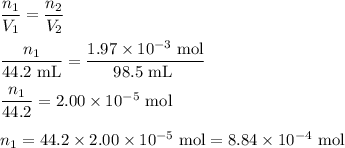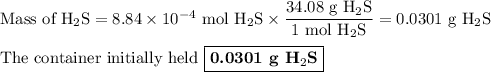Chemistry, 28.06.2019 05:50 dinarussell74
Hydrogen sulfide gas (h2s) is a highly toxic gas that is responsible for the smell of rotten eggs. the volume of a container of hydrogen sulfide is 44.2ml. after the addition of more hydrogen sulfide, the volume increases to 98.5ml under constant pressure and temperature. the container now holds 1.97×10−3mol of the gas. how many grams of hydrogen sulfide were in the container initially? give your answer in three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
Hydrogen sulfide gas (h2s) is a highly toxic gas that is responsible for the smell of rotten eggs. t...
Questions




Mathematics, 19.03.2020 06:11





Biology, 19.03.2020 06:11

English, 19.03.2020 06:11


Mathematics, 19.03.2020 06:12



Mathematics, 19.03.2020 06:12


History, 19.03.2020 06:12