

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
There are three different possible structures (known as isomers) of a dibromoethene molecule, c2h2br...
Questions

Advanced Placement (AP), 08.02.2021 19:30



Mathematics, 08.02.2021 19:30



Social Studies, 08.02.2021 19:30


Mathematics, 08.02.2021 19:30

Biology, 08.02.2021 19:30


Mathematics, 08.02.2021 19:30



History, 08.02.2021 19:30



Mathematics, 08.02.2021 19:30


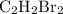 .
.


