
Chemistry, 28.06.2019 03:00 aidentrooper8629
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric acid. if the percent yield of the reaction was 82.15%, what was the actual amount of calcium chloride formed? caco3 + hcl → cacl2 + co2 + h2o 105.3 grams 101.1 grams 95.6 grams 86.5 grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric ac...
Questions


Mathematics, 04.03.2021 02:20

Arts, 04.03.2021 02:20

Chemistry, 04.03.2021 02:20



Mathematics, 04.03.2021 02:20

Mathematics, 04.03.2021 02:20

Biology, 04.03.2021 02:20


Biology, 04.03.2021 02:20

Mathematics, 04.03.2021 02:20


Mathematics, 04.03.2021 02:20

World Languages, 04.03.2021 02:20




Mathematics, 04.03.2021 02:20

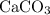 ?
?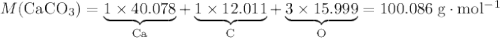 .
.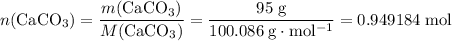 .
.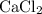 will be produced?
will be produced?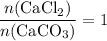 .
.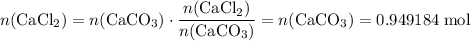 .
.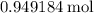 of
of 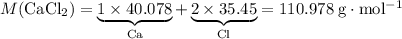 .
.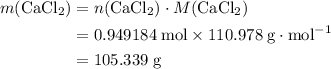 .
.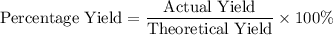 .
. .
.

