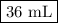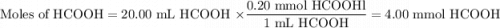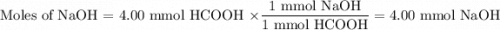You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you will titrate this with a 0.1105 m naoh. if you add 20.00 ml of hcooh to the beaker before titrating, approximately what volume of naoh will be required to reach the end point? view available hint(s) you are given a solution of (formic acid) with an approximate concentration of 0.20 and you will titrate this with a 0.1105 . if you add 20.00 of to the beaker before titrating, approximately what volume of will be required to reach the end point? 11.1 ml 20.0 ml 72.4 ml 36.2 ml

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you...
Questions

Mathematics, 07.12.2020 06:30




Mathematics, 07.12.2020 06:30

Mathematics, 07.12.2020 06:30

History, 07.12.2020 06:30


Mathematics, 07.12.2020 06:30




Mathematics, 07.12.2020 06:30

Mathematics, 07.12.2020 06:30


English, 07.12.2020 06:30

English, 07.12.2020 06:30


Mathematics, 07.12.2020 06:30

Mathematics, 07.12.2020 06:30