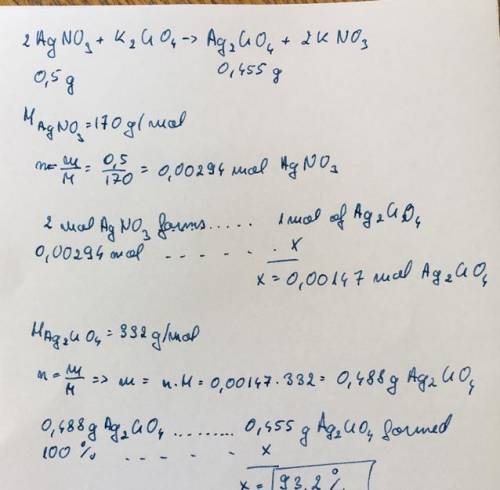Chemistry, 27.06.2019 07:20 maxi12312345
3. calculate the percent yield of silver chromate if 0.455 grams of silver chromate are producedfrom 0.500 grams of silver nitrate.2 agno3 + k2cro4 -> ag2 cro 4 + 2 kno3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
3. calculate the percent yield of silver chromate if 0.455 grams of silver chromate are producedfrom...
Questions





Advanced Placement (AP), 15.01.2021 21:20


Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

Health, 15.01.2021 21:20


Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20


Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20


Mathematics, 15.01.2021 21:20