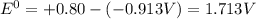Based on the sign of e cell, classify these reactions as spontaneous or non spontaneous as written.? assume standard conditions. ni^2+ (aq) + s^2- (aq) > + ni (s) s (s) (nonspontaneous)? pb^2+ (aq) +h2 (g) > pb (s) +2h^+ (aq) (nonspontaneous)? 2ag^+ (aq) + cr(s) > 2 ag (s) +cr^2+ (aq) (spontaneous? ) are these correct?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Based on the sign of e cell, classify these reactions as spontaneous or non spontaneous as written.?...
Questions



Mathematics, 08.01.2022 08:20



English, 08.01.2022 08:20


Social Studies, 08.01.2022 08:20


Mathematics, 08.01.2022 08:20

Mathematics, 08.01.2022 08:20



Mathematics, 08.01.2022 08:30

Mathematics, 08.01.2022 08:30


Social Studies, 08.01.2022 08:30


English, 08.01.2022 08:30

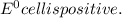
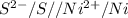
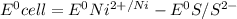
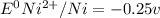
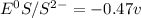
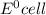 = - 0.25 - (-0.47) = 0.22 v
= - 0.25 - (-0.47) = 0.22 v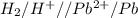
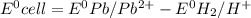
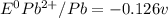
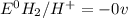
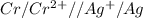
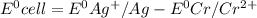
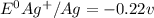
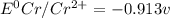
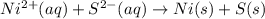 : non spontaneous
: non spontaneous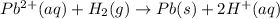 : non spontaneous
: non spontaneous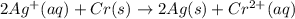 : spontaneous
: spontaneous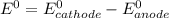
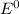 are standard reduction potentials.
are standard reduction potentials.
![E^0_{[Ni^{2+}/Ni]}=-0.25V](/tpl/images/0340/4979/2bf3e.png)
![E^0_{[S^{2-}/S]}=0.407VV](/tpl/images/0340/4979/b9faa.png)
![E^0=E^0_{[Ni^{2+}/Ni]}- E^0_{[S^{2-}/S]}](/tpl/images/0340/4979/7165d.png)
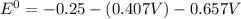
![E^0_{[Pb^{2+}/Pb]}=-0.13](/tpl/images/0340/4979/2e29f.png)
![E^0_{[H^{+}/H_2]}=0V](/tpl/images/0340/4979/311ce.png)
![E^0=E^0_{[Pb^{2+}/Pb]}- E^0_{[H^{+}/H_2]}](/tpl/images/0340/4979/1bbe8.png)
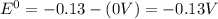
![E^0_{[Ag^{+}/Ag]}=+0.80V](/tpl/images/0340/4979/76e17.png)
![E^0_{[Cr^{2+}/Cr]}=-0.913V](/tpl/images/0340/4979/0a8f3.png)
![E^0=E^0_{[Ag^{+}/Ag]}- E^0_{[Cr^{2+}/Cr]}](/tpl/images/0340/4979/5f991.png)