
Chemistry, 18.11.2019 13:31 ansarishaheer2888
Given the balanced equation representing a reaction: 4al(s) + 3o2(g) → 2al2o3(s) how many moles of al(s) react completely with 4.50 moles of o2(g) to produce 3.00 moles of al2o3(s)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2
You know the right answer?
Given the balanced equation representing a reaction: 4al(s) + 3o2(g) → 2al2o3(s) how many moles of...
Questions


English, 05.05.2020 22:21



Mathematics, 05.05.2020 22:21

Chemistry, 05.05.2020 22:21


Mathematics, 05.05.2020 22:21


Mathematics, 05.05.2020 22:21

Mathematics, 05.05.2020 22:21




History, 05.05.2020 22:21

Mathematics, 05.05.2020 22:21

Mathematics, 05.05.2020 22:21

Arts, 05.05.2020 22:21


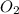 :
: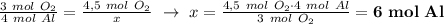
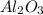 , keeping constant the proportion of both substances.
, keeping constant the proportion of both substances.


