
Chemistry, 26.06.2019 18:50 christianfielding336
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is produced. b. co2 concentration increases. c. the equilibrium is pushed in the direction of reactants. d. nothing
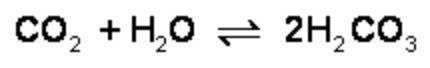

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1
You know the right answer?
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is pro...
Questions


Mathematics, 09.11.2020 22:10





History, 09.11.2020 22:10

Chemistry, 09.11.2020 22:10


Mathematics, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10





English, 09.11.2020 22:10


History, 09.11.2020 22:10

Social Studies, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10



