
Chemistry, 25.06.2019 19:50 summerdooleyu
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is produced. b. co2 concentration increases. c. the equilibrium is pushed in the direction of reactants. d. nothing
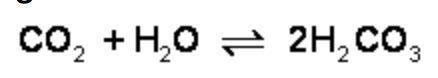

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

You know the right answer?
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is pro...
Questions


Mathematics, 16.04.2021 21:20







Mathematics, 16.04.2021 21:20

Chemistry, 16.04.2021 21:20





Mathematics, 16.04.2021 21:20



Mathematics, 16.04.2021 21:20




