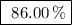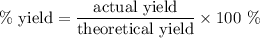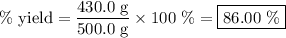Chemistry, 25.06.2019 08:00 saadizak7098
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction meant to fill an order for a customer who would like to purchase 500.0g of ammonia. when the reaction is complete, the company finds they produced only 430.0g of ammonia. what it their percent yield for that reaction? 70.00%86.00%116.3%93.00%

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction...
Questions

Social Studies, 05.05.2020 10:03

Mathematics, 05.05.2020 10:03



Mathematics, 05.05.2020 10:03

Chemistry, 05.05.2020 10:03

History, 05.05.2020 10:03



Mathematics, 05.05.2020 10:03



Mathematics, 05.05.2020 10:03


Chemistry, 05.05.2020 10:03

English, 05.05.2020 10:03

Mathematics, 05.05.2020 10:03

Physics, 05.05.2020 10:03

Spanish, 05.05.2020 10:03