
Chemistry, 25.06.2019 03:10 timrelawan06
How much heat is released when you condense 93.9 g of water vapor? show work ❤️

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

You know the right answer?
How much heat is released when you condense 93.9 g of water vapor? show work ❤️...
Questions

Social Studies, 31.03.2021 03:50


Mathematics, 31.03.2021 03:50


History, 31.03.2021 03:50






Computers and Technology, 31.03.2021 03:50


Biology, 31.03.2021 03:50




History, 31.03.2021 03:50


Mathematics, 31.03.2021 03:50

Mathematics, 31.03.2021 03:50

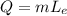
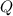 = heat energy,
= heat energy, 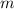 = mass of water and
= mass of water and 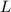 = latent heat of evaporation.
= latent heat of evaporation.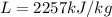 and the mass of the water is
and the mass of the water is 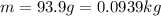 .
.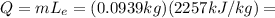 211.9 J
211.9 J

