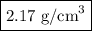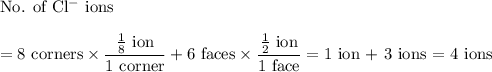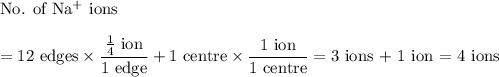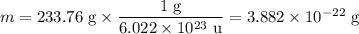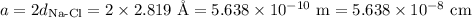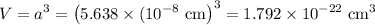Chemistry, 24.06.2019 18:10 makikorising1226
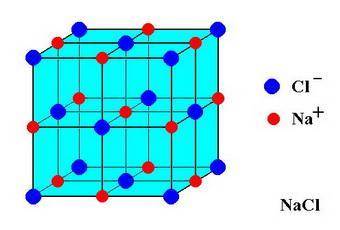
In sodium chloride, the distance between the center of the sodium ion and the centerof an adjacent chloride ion is 2.819 angstroms. calculate the density in g/cm3of an ideal nacl crystal from this information and what you learned from this lab. hints: to calculate mass, determine how many equivalent ions are in a unit cell. to determinevolume of the unit cell, start by determining the length of on side of the unit cell.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

You know the right answer?
In sodium chloride, the distance between the center of the sodium ion and the centerof an adjacent c...
Questions

Mathematics, 26.03.2020 22:00

Mathematics, 26.03.2020 22:00


History, 26.03.2020 22:00

English, 26.03.2020 22:00

Mathematics, 26.03.2020 22:00

Mathematics, 26.03.2020 22:00

Mathematics, 26.03.2020 22:00





Mathematics, 26.03.2020 22:00

History, 26.03.2020 22:00

Mathematics, 26.03.2020 22:00