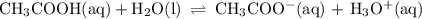Chemistry, 24.06.2019 18:10 terrieldixon
Consider a buffer solution containing ch3cooh and ch3coo-, with an equilibrium represented by: ch3cooh(aq) + h2o (l) ←→ h3o+ (aq) + ch3coo- (aq) describe what occurs if a strong acid such as hno3 is added to the system, including an explanation of the direction of the equilibrium shift. describe what occurs if a strong base such as koh is added, including an explanation of the direction of the equilibrium shift.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1
You know the right answer?
Consider a buffer solution containing ch3cooh and ch3coo-, with an equilibrium represented by: ch3c...
Questions


History, 12.04.2021 21:10


History, 12.04.2021 21:10


Mathematics, 12.04.2021 21:10



History, 12.04.2021 21:10


Biology, 12.04.2021 21:10


Mathematics, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10


Mathematics, 12.04.2021 21:10

Biology, 12.04.2021 21:10


Mathematics, 12.04.2021 21:10