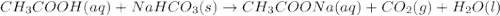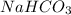Chemistry, 24.06.2019 15:20 janakibubbles7711
Vinegar is an aqueous solution of acetic acid (hch3coo). when vinegar reacts with dry baking soda (nahco3), the result is an aqueous solution of sodium acetate (nach3coo), liquid water, and carbon-dioxide gas, which bubbles out of the solution. which chemical equation correctly represents this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Vinegar is an aqueous solution of acetic acid (hch3coo). when vinegar reacts with dry baking soda (n...
Questions








Mathematics, 21.08.2019 21:20

Spanish, 21.08.2019 21:20


History, 21.08.2019 21:20


Advanced Placement (AP), 21.08.2019 21:20



History, 21.08.2019 21:20

French, 21.08.2019 21:30

Mathematics, 21.08.2019 21:30

Mathematics, 21.08.2019 21:30