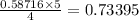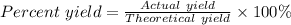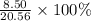Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1
You know the right answer?
When 10.0 g of nh3 reacts, the actual yield of n2 is 8.50 g. what is the percent yield? 4 nh3 (g) +...
Questions


Mathematics, 21.05.2021 22:20

Social Studies, 21.05.2021 22:20

Mathematics, 21.05.2021 22:20

Mathematics, 21.05.2021 22:20

Mathematics, 21.05.2021 22:20



Mathematics, 21.05.2021 22:20



Chemistry, 21.05.2021 22:20

Spanish, 21.05.2021 22:20

Mathematics, 21.05.2021 22:20



Physics, 21.05.2021 22:20

Mathematics, 21.05.2021 22:20


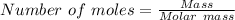
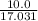
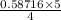 moles of N₂
moles of N₂