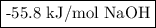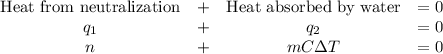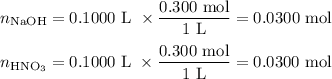A100.0 ml sample of 0.300 m naoh is mixed with a 100.0 ml sample of 0.300 m hno3 in a coffee cup calorimeter. if both solutions were initially at 35.00°c and the temperature of the resulting solution was recorded as 37.00°c, determine the δh°rxn (in units of kj/mol naoh) for the neutralization reaction between aqueous naoh and hcl. assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water. - 34.4 kj/mol naoh -169 kj/mol naoh -55.7 kj/mol naoh -27.9 kj/mol naoh -16.7 kj/mol naoh

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
A100.0 ml sample of 0.300 m naoh is mixed with a 100.0 ml sample of 0.300 m hno3 in a coffee cup cal...
Questions

English, 10.09.2021 04:20



Mathematics, 10.09.2021 04:20


Mathematics, 10.09.2021 04:20

Chemistry, 10.09.2021 04:20

Social Studies, 10.09.2021 04:20

Mathematics, 10.09.2021 04:20


Mathematics, 10.09.2021 04:20

History, 10.09.2021 04:20

Social Studies, 10.09.2021 04:20


Mathematics, 10.09.2021 04:20

English, 10.09.2021 04:20



Mathematics, 10.09.2021 04:20

History, 10.09.2021 04:20