
Chemistry, 24.06.2019 06:20 dtovar4922
How many moles are equivalent to 3.58x1023 formula units of zncl2? a. 0.555 mol zncl2 c. 0.621 mol zncl2 b. 1 mol zncl2 d. 0.595 mol zncl2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
How many moles are equivalent to 3.58x1023 formula units of zncl2? a. 0.555 mol zncl2 c. 0.621 mol...
Questions
















Chemistry, 24.06.2019 01:30

English, 24.06.2019 01:30


Mathematics, 24.06.2019 01:30

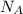 or equivalently,
or equivalently, 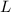 :
: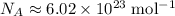 .
.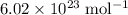 constituent particles (Wikipedia) in each mole of a substance. In this case,
constituent particles (Wikipedia) in each mole of a substance. In this case, 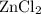 is the substance, and
is the substance, and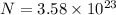 .
.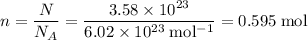 .
.

