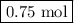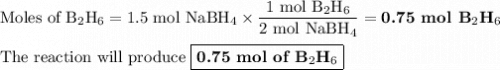Chemistry, 23.06.2019 07:20 msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2...
Questions

History, 11.07.2019 15:40





Arts, 11.07.2019 15:40

Chemistry, 11.07.2019 15:40


Biology, 11.07.2019 15:40

Biology, 11.07.2019 15:40

Mathematics, 11.07.2019 15:40

Mathematics, 11.07.2019 15:40


Mathematics, 11.07.2019 15:40

History, 11.07.2019 15:40

History, 11.07.2019 15:40


Social Studies, 11.07.2019 15:40

Chemistry, 11.07.2019 15:40

English, 11.07.2019 15:40