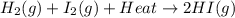The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this r...
Questions

English, 03.07.2019 02:30


Biology, 03.07.2019 02:30

History, 03.07.2019 02:30


Business, 03.07.2019 02:30

History, 03.07.2019 02:30

Mathematics, 03.07.2019 02:30


Mathematics, 03.07.2019 02:30

Biology, 03.07.2019 02:30

Mathematics, 03.07.2019 02:30



History, 03.07.2019 02:30

Mathematics, 03.07.2019 02:30



Mathematics, 03.07.2019 02:30