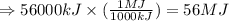Chemistry, 22.06.2019 18:00 rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the...
Questions

Biology, 05.05.2020 23:24


Mathematics, 05.05.2020 23:24



Mathematics, 05.05.2020 23:24




Mathematics, 05.05.2020 23:24

English, 05.05.2020 23:24


Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24


History, 05.05.2020 23:24

Chemistry, 05.05.2020 23:24

History, 05.05.2020 23:24


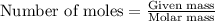
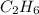 = 12 kg = 12000 g (Conversion factor: 1 kg = 1000 g)
= 12 kg = 12000 g (Conversion factor: 1 kg = 1000 g)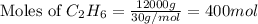
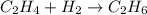
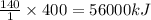 of heat.
of heat.