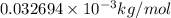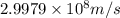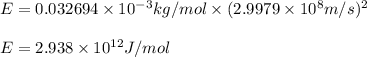Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of...
Questions

Biology, 04.05.2021 19:10

Advanced Placement (AP), 04.05.2021 19:10

Mathematics, 04.05.2021 19:10

Mathematics, 04.05.2021 19:10

English, 04.05.2021 19:10


Mathematics, 04.05.2021 19:10


Mathematics, 04.05.2021 19:10

Mathematics, 04.05.2021 19:10


English, 04.05.2021 19:10

English, 04.05.2021 19:10

Arts, 04.05.2021 19:10

Mathematics, 04.05.2021 19:10

Mathematics, 04.05.2021 19:10


Mathematics, 04.05.2021 19:10

Mathematics, 04.05.2021 19:10

Mathematics, 04.05.2021 19:10

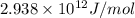
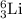
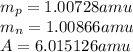
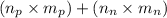
![M=[(3\times 1.00728)+(3\times 1.00866)]=6.04782amu](/tpl/images/0004/4360/7bbb8.png)
![\Delta m=M-A\\\Delta m=[6.04782-6.015126)]=0.032694amu=0.032694g/mol](/tpl/images/0004/4360/0877a.png)
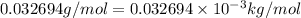

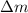 = Mass defect =
= Mass defect =