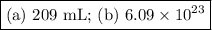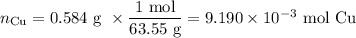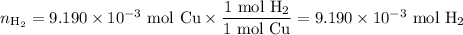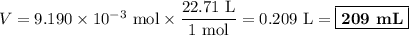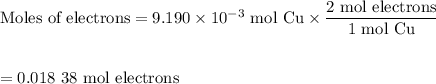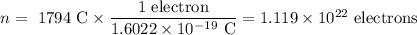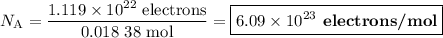An acidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. (a) what is the gas produced at the cathode and what is its volume at stp? name of gas volume of gas webassign will check your answer for the correct number of significant figures. l (b) given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
An acidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused...
Questions

Mathematics, 29.10.2020 17:50

History, 29.10.2020 17:50

Chemistry, 29.10.2020 17:50


English, 29.10.2020 17:50

Mathematics, 29.10.2020 17:50

Mathematics, 29.10.2020 17:50



English, 29.10.2020 17:50

Arts, 29.10.2020 17:50


History, 29.10.2020 17:50



English, 29.10.2020 17:50

English, 29.10.2020 17:50