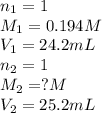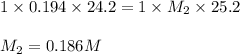An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of h...
Questions


Mathematics, 11.07.2021 01:00








Mathematics, 11.07.2021 01:00





Mathematics, 11.07.2021 01:00

Business, 11.07.2021 01:00



Mathematics, 11.07.2021 01:00

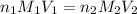
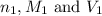 are the n-factor, molarity and volume of acid which is HBr
are the n-factor, molarity and volume of acid which is HBr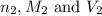 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.