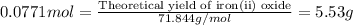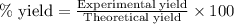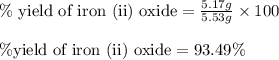Chemistry, 26.10.2019 11:43 Kathryn014
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yields 5.17 grams of iron(ii) oxide . iron ( s ) + oxygen ( g ) iron(ii) oxide ( s ) what is the theoretical yield of iron(ii) oxide ? 21.6 grams what is the percent yield for this reaction ? 85 %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 15:00
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b.liquid c.gas
Answers: 2
You know the right answer?
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yield...
Questions

Mathematics, 17.02.2021 02:40


Mathematics, 17.02.2021 02:40


Arts, 17.02.2021 02:40



Mathematics, 17.02.2021 02:40

Mathematics, 17.02.2021 02:40


Mathematics, 17.02.2021 02:40

Mathematics, 17.02.2021 02:40

Mathematics, 17.02.2021 02:40

Mathematics, 17.02.2021 02:40

Mathematics, 17.02.2021 02:40

Business, 17.02.2021 02:40




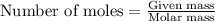 ....(1)
....(1)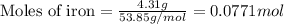
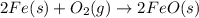
 of iron (ii) oxide
of iron (ii) oxide