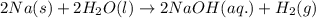Chemistry, 21.06.2019 15:50 algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3


Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hy...
Questions

Mathematics, 06.05.2021 19:30

Mathematics, 06.05.2021 19:30





Mathematics, 06.05.2021 19:30



Mathematics, 06.05.2021 19:30

Mathematics, 06.05.2021 19:30

History, 06.05.2021 19:30

Social Studies, 06.05.2021 19:30


Geography, 06.05.2021 19:30


Social Studies, 06.05.2021 19:30

Biology, 06.05.2021 19:30