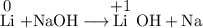How does the oxidation state of na change in the following reaction?
li(s) + naoh(aq) → lioh(a...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Questions

Arts, 19.12.2020 20:40


Business, 19.12.2020 20:40

Arts, 19.12.2020 20:40

Mathematics, 19.12.2020 20:40

Chemistry, 19.12.2020 20:40





Advanced Placement (AP), 19.12.2020 20:40


English, 19.12.2020 20:40




Medicine, 19.12.2020 20:40

Mathematics, 19.12.2020 20:40


Mathematics, 19.12.2020 20:40