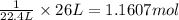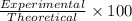Chemistry, 25.12.2019 11:31 jamarstand
Hydrogen gas (a potential future fuel) can be formed by the reaction of methane with water according to the following equation: ch4(g)+h2o(g)→co(g)+3h2(g) in a particular reaction, 26.0 l of methane gas (measured at a pressure of 734 torr and a temperature of 25 ∘c) is mixed with 23.0 l of water vapor (measured at a pressure of 700 torr and a temperature of 125 ∘c). the reaction produces 26.0 l of hydrogen gas measured at stp. part a what is the percent yield of the reaction? %

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
Hydrogen gas (a potential future fuel) can be formed by the reaction of methane with water according...
Questions



Mathematics, 23.09.2019 09:30




Chemistry, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30


Mathematics, 23.09.2019 09:30


English, 23.09.2019 09:30


Biology, 23.09.2019 09:30

Biology, 23.09.2019 09:30




Mathematics, 23.09.2019 09:30

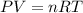
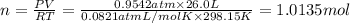
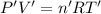
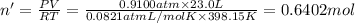
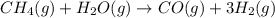
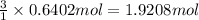 of hydrogen gas
of hydrogen gas