
The explosive nitroglycerin (c3h5n3o9) decomposes rapidly upon ignition or sudden impact according to the following balanced equation: 4 c3h5n3o9 (l) → 12 co2 (g) + 10 h2o (g) + 6 n2 (g) + o2 (g) δrxnho = −5678 kj calculate the standard enthalpy of formation (δfho) for nitroglycerin. the enthalpy of formation of co2 (g) is -393.5 kj/mol. the enthalpy of formation of h2o (g) is -241.8 kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
The explosive nitroglycerin (c3h5n3o9) decomposes rapidly upon ignition or sudden impact according t...
Questions




Business, 15.02.2021 02:20



Mathematics, 15.02.2021 02:20

History, 15.02.2021 02:20

Mathematics, 15.02.2021 02:20





Mathematics, 15.02.2021 02:20

Business, 15.02.2021 02:20

Mathematics, 15.02.2021 02:20



Mathematics, 15.02.2021 02:20

Mathematics, 15.02.2021 02:20

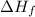 for
for 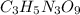 in the reaction is -365.5 kJ/mol.
in the reaction is -365.5 kJ/mol.![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0445/1737/18a63.png)
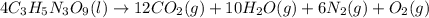
![\Delta H_{rxn}=[(12\times \Delta H_f_{(CO_2)})+(10\times \Delta H_f_{(H_2O)})+(6\times \Delta H_f_{(N_2)})+(1\times \Delta H_f_{(O_2)})]-[(4\times \Delta H_f_{(C_3H_5N_3O_9)})]](/tpl/images/0445/1737/c0f93.png)
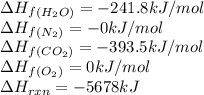
![-5678=[(12\times (-393.5))+(10\times (-241.8))+(6\times (0))+(1\times (0))]-[(4\times \Delta H_f_{(C_3H_5N_3O_9)})]\\\\\Delta H_f_{(C_3H_5N_3O_9)}=-365.5kJ/mol](/tpl/images/0445/1737/dcb72.png)


