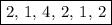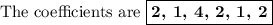In acidic solution, the nitrate ion can be used to react with a number of metal ions. one such reaction is no3−(aq)+sn2+(aq)→no2(aq)+sn4+(aq) since this reaction takes place in acidic solution, h2o(l) and h+(aq) will be involved in the reaction. places for these species are indicated by the blanks in the following restatement of the equation: no3−(aq)+sn2+(aq)+ −−−→no2(aq)+sn4+(aq)+ −−− part a what are the coefficients of the reactants and products in the balanced equation above? remember to include h2o(l) and h+(aq) in the appropriate blanks. your answer should have six terms. enter the equation coefficients in order separated by commas (e. g., 2,2,1,4,4,3). include coefficients of 1, as required, for grading purposes.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

You know the right answer?
In acidic solution, the nitrate ion can be used to react with a number of metal ions. one such react...
Questions


Chemistry, 06.04.2021 18:40

Social Studies, 06.04.2021 18:40


English, 06.04.2021 18:40


History, 06.04.2021 18:40

Mathematics, 06.04.2021 18:40

Mathematics, 06.04.2021 18:40

Mathematics, 06.04.2021 18:40



Health, 06.04.2021 18:50





Mathematics, 06.04.2021 18:50

Mathematics, 06.04.2021 18:50