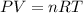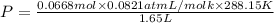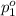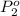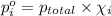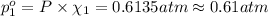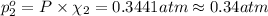Chemistry, 13.10.2019 01:10 sabrinamarie391
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the mole fraction of n2. express your answer using two significant figures. x1 x 1 = nothing request answer part b calculate the mole fraction of o2. express your answer using two significant figures. x2 x 2 = nothing request answer part c calculate the partial pressure of n2. express your answer using two significant figures. p1 p 1 = nothing atm request answer part d calculate the partial pressure of o2. express your answer using two significant figures. p2 p 2 = nothing atm request answer provide feedback

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the m...
Questions

Social Studies, 26.05.2021 22:20


Mathematics, 26.05.2021 22:20

Mathematics, 26.05.2021 22:20


Health, 26.05.2021 22:20

Mathematics, 26.05.2021 22:20

Social Studies, 26.05.2021 22:20

Mathematics, 26.05.2021 22:20


Mathematics, 26.05.2021 22:20

Mathematics, 26.05.2021 22:20


Mathematics, 26.05.2021 22:20

History, 26.05.2021 22:20

Mathematics, 26.05.2021 22:20

Physics, 26.05.2021 22:20



Mathematics, 26.05.2021 22:20

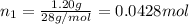
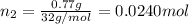
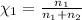
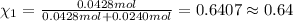
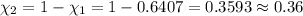
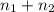 = 0.0428 mol + 0.0240 mol = 0.0668 mol
= 0.0428 mol + 0.0240 mol = 0.0668 mol