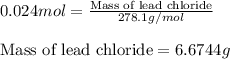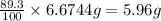An aqueous solution containing 7.96 g7.96 g of lead(ii) nitrate is added to an aqueous solution containing 6.82 g6.82 g of potassium chloride. enter the balanced chemical equation for this reaction. be sure to include all physical states. balanced chemical equation: what is the limiting reactant? potassium chloride lead(ii) nitrate the percent yield for the reaction is 89.3%89.3% . how many grams of precipitate is recovered? precipitate recovered: gg how many grams of the excess reactant remain? excess reactant remaining: g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
An aqueous solution containing 7.96 g7.96 g of lead(ii) nitrate is added to an aqueous solution cont...
Questions


Mathematics, 15.02.2021 22:40

Mathematics, 15.02.2021 22:40

Biology, 15.02.2021 22:40



Biology, 15.02.2021 22:40


Mathematics, 15.02.2021 22:40


English, 15.02.2021 22:40

Mathematics, 15.02.2021 22:40

Mathematics, 15.02.2021 22:40


Mathematics, 15.02.2021 22:50


Mathematics, 15.02.2021 22:50


Mathematics, 15.02.2021 22:50

Mathematics, 15.02.2021 22:50

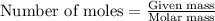 ....(1)
....(1)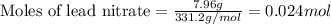
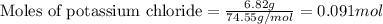
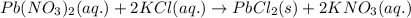
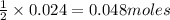 of potassium chloride
of potassium chloride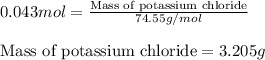
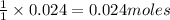 of lead chloride
of lead chloride