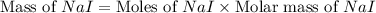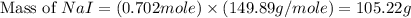Chemistry, 15.11.2019 14:31 ejfleck3655
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solution containing sodium iodide. 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) how many grams of sodium iodide, nai, nai, must be used to produce 89.1 g89.1 g of iodine, i2? i2? mass: g nai

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1


Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solu...
Questions


History, 10.05.2021 02:20

Arts, 10.05.2021 02:20

Mathematics, 10.05.2021 02:20




Mathematics, 10.05.2021 02:20

Mathematics, 10.05.2021 02:20

Mathematics, 10.05.2021 02:20


Mathematics, 10.05.2021 02:20

Computers and Technology, 10.05.2021 02:20



Mathematics, 10.05.2021 02:30




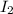 = 89.1 g
= 89.1 g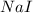 = 149.89 g/mole
= 149.89 g/mole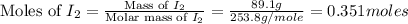
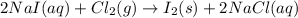
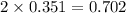 moles of
moles of